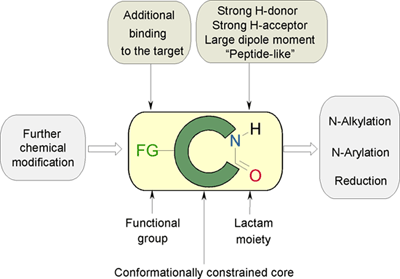
STEREOCHEMISTRY
A. Enantiomers and the tetrahedral carbon
Try to pay attention CH3X compounds, CH2XY, and CHXYZ. On the left side is 3 molecule and the right were the mirror image of the three compounds. CH3X compounds, and CH2XY is identical with the shadow of the mirror. If you make a molecular model of each molecule and the molecular mirror image, it will get that you can squeeze a molecule with a mirror image (superimposed). Unlike CH3X, and CH2XY, CHXYZ not identical a mirror image. You can not do superimposed molecular models and molecular models of the mirror image, just as you squeeze your right hand with your left hand. Maybe you can squeeze 2 subtituennya, eg X and Y, but the H and Z will each opposite. Similarly, if the coincident H and Z, X and Y will also of each other.

Mirror-image molecules that can not coincide called enantiomers (Greek enantio means contrary / opposite). Enantiomers related to carbon tetrahedral. Suppose that lactic acid (acid 2-hidroksipropanoat) that a pair of enantiomers due to having four groups different (-OH,-H,-CH3,-CO2H) on the carbon atom as center. Enantiomers are called (+)-lactic acid and (-)-acid lactate.
You do not molecule may squeeze (+) lactic acid in the molecule (-) lactic acid. If the two groups may coincide, for example, H and - COOH, the two other groups will not be able to coincide.
B. Chirality
If a molecule can not coincide with means that the mirror image enantiomers of both compounds are called
Chiral / chiral (ky-ral Greek cheir, meaning "hand").
Which has the symmetry of the molecule in various likely to be identical in conformation with the shadow
mirror and is therefore a compound nonkiral or so-called achiral. Most, though not all, the cause of the chirality due to a compound that binds to the carbon atom 4 different groups. Carbon atom is termed as a center of chirality (chirality centers). Note, the chirality is the nature of the whole molecule, where the center of chirality is a characteristic structure cause chirality. Chirality is not only determined by the difference
atom is bonded directly to carbon, but the difference in the fourth groups bound to carbon as a chiral center. Such as 5 - bromodekana a chiral compound as it binds four different groups.
Br
|
CH3CH2CH2CH2CH2-C-CH2CH2CH2CH3
|
H
5-Bromodekana
(Chiral)
Substituent butyl substituents similar to the nipple. However, both Another example is not the same metilsikloheksana. whether the molecule The chiral? Metilsikloheksana an achiral molecule, because does not have four different groups attached to one carbon. Atoms C2, C3, C4, C5, and C6 atoms is clearly not chiral because it has two H atoms are identical (-CH2-). C1 atoms bonded to H, CH3, C2 and C6, in which
C2 atom in the C3-C4 terkat while bound to the C5-C6 C4. by because the C2-C3-C4-C5 is identical to the C6 atom C1-C4 is not the atom chiral. So overall, not a chiral molecule compounds metilsikloheksana.
C. Optical activity
In addition to the chirality, the structures of the couple enantiomer is the same. Therefore, almost all physical properties also the same chemical. For example, each have a pure enantiomer melting point and boiling point with the same partner. only There are two different properties for the enantiomer-enantiomer in a pair of enantiomers, namely:
- Inter-action with other chiral compounds
- Inter-action with polarized light
Studies on the stereochemistry at the beginning of the nineteenth century by French scientist, Jean Batiste Biot. Biot discovered the nature of light polarized field (plane-polarized light). A light beam is composed of electromagnetic waves that oscillate at infinite field at an angle perpendicular to the direction of propagation
wave. When a light beam through the polarizer, only isolation waves in a field that can be passed so-called polarized light field. Apparently some of the optically active molecules can rotate plane of polarization. Optically active molecules that rotate the plane of polarization to the right (clockwise) is called dextrorotatory (dextrorotatory) or given notation (+). Conversely, when the rotating field of optically active molecules
polarization to the left (counterclockwise) is said to be levorotatori or negetif notation (-). Specific rotation, [α] D, of a compound can be defined as the rotation of light (λ = 589 nm) is generated when the sample
D. Specific configuration rules
Cahn Ingold Prelog rules
Rule 1: Consider the four atoms directly attached to the chirality center and make priorities based on the decrease in number atom. Atoms that have the priority to occupy the highest atomic number first, while the atom that has a low atomic number (usually hydrogen) occupies the fourth priority.
Rule 2: If the creation of priority can not use the rule 1, compare the number of atoms at the second atom of each substituent, followed on the third atom, and so on until found differences in their atomic numbers so that it can be made a priority.
Rule 3: Multibonding atom is equal to the number of atoms with single bonds. Having made a priority in the four substituents attached directly to the chiral carbon centers, we can determine the configuration stereochemistry by changing the orientation of the molecules so that the four priorities of substituents are made away from us. The remaining three substituents will look like steer a car. The next step made toward
Turn the car steer us in the order of priority until finally the third priority. If it turns out that steer the direction
we make a clockwise (clockwise) then said to be the center of chirality has the R configuration (the letter R comes from the Latin rectus which means "right"). If we steer the direction opposite to the direction clockwise (counterclockwise) is said to be the center of chirality has the S configuration (the letter S comes from the Latin sinister which means "left").
E. Diastereomers
Diastereomers are stereoisomers that are not shadows mirror. Chiral diastereomers having the opposite configuration at the chiral centers but has some configuration The same with the others. As a comparison, enantiomer which has the opposite configuration at all chiral centers.
F. Meso compounds
Mirror-image structure 2R, 3R and 2S, 3S is not identical but a pair of enantiomers. if the observed actually, the structure of 2R, 3R and 2S, 3S is identical if one structure is rotated 180 °.
The structure of 2R, 3S and 2S, 3R are identical because the molecules it has a plane of symmetry so that the achiral. plane of symmetry cut on the C2-C3 bond to half a the mirror image of the next half.
The above compound is achiral, but contains two central Chiral called meso compounds.
G. Molecules that have more than two
Chiral Center
Apparently a chiral center in one molecule give two stereoisomers (a pair of enantiomers) and two chiral centers in one molecule to give a maximum of 4 stereoisomers or 2 pairs of enantiomers. In general, a molecule with n center has a maximum of 2n chiral stereoisomer or 2n-1 pairs enantiomer, although it may be less because of possible several stereoisomers are meso compounds. Examples of cholesterol contain chiral centers 8, 28 = 256 possible stereoisomers, although some of them are too complicated to exist, there is only one found in nature.
or